Gel electrophoresis is a method employed to segregate DNA fragments (or other large molecules like RNA and proteins) by taking into account their size and charge. The process entails passing an electric current through a gel containing the analyzed molecules. Due to variations in size and charge, the molecules will migrate through the gel in distinct directions or at different velocities, facilitating their separation from each other.
The charge-to-mass ratio of all DNA molecules is uniform, which leads to the separation of DNA fragments solely based on their size during gel electrophoresis. We can observe the number of distinct DNA fragments in a sample and compare their relative sizes by utilizing electrophoresis. Additionally, we can determine the precise size of a DNA segment by comparing it to a standard "yardstick" consisting of DNA fragments with established sizes.
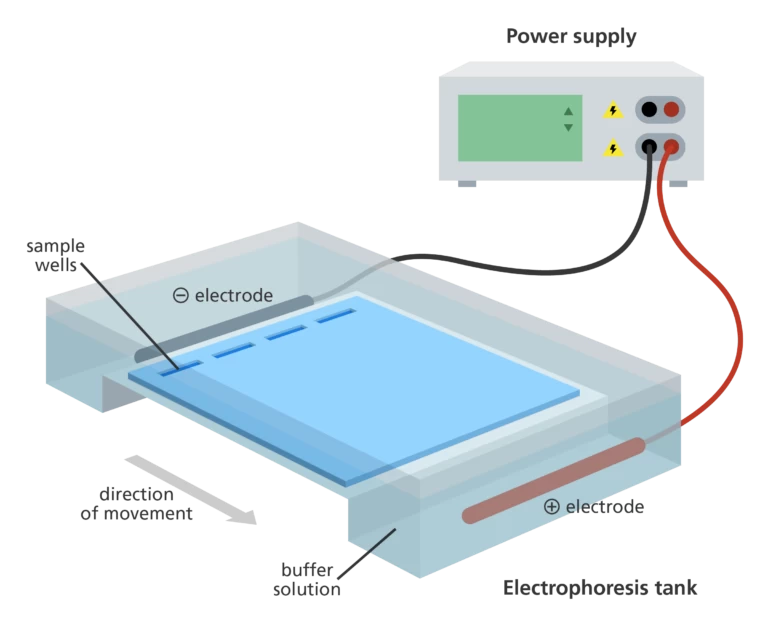
The gel electrophoresis setup comprises a gel, typically composed of agar or polyacrylamide, and an electrophoretic chamber that is commonly a rigid plastic box or tank. Positioned at one end of the chamber is the cathode, which serves as the negative terminal, while the anode, functioning as the positive terminal, is situated at the opposite end. The gel encompasses a series of wells located near the cathode. The gel is carefully positioned inside the chamber and covered with a buffer solution. Using a pipette, the samples are loaded into the wells.
To initiate the process, the chamber is connected to a power supply, which, upon activation, applies an electric field to the buffer. This electric field induces the migration of negatively charged molecules, such as DNA and RNA (proteins require treatment with a detergent to acquire a negative charge), through the gel towards the anode. The movement of these molecules is influenced by the porous gel matrix, resulting in larger and heavier molecules migrating at a relatively slower pace compared to smaller and lighter molecules. The density of pores within the gel and the gel material itself further impact the rate at which the molecules migrate.
In many cases, a stained "ladder" or marker containing molecules with known molecular weights is run alongside the experimental samples, serving as a reference for size determination. The marker is visually tracked as it moves through the gel, aided by the presence of a dye. Experimental samples are typically also dyed to enhance their visualization. A commonly used dye for DNA samples is ethidium bromide, which exhibits fluorescence under ultraviolet light, facilitating clear visualization during the gel electrophoresis process.
As implied by its name, gel electrophoresis employs a gel-like substance known as a gel. Gels used for DNA separation are commonly composed of agarose, a polysaccharide. Agarose is typically obtained in the form of dry, powdered flakes. To create the gel, the agarose is heated in a buffer solution containing water and salts. Upon cooling, the agarose solidifies into a slightly soft gel structure. On a molecular level, the gel consists of a matrix comprising agarose molecules that are interconnected by hydrogen bonds, resulting in the formation of small pores. At one end of the gel, there are well-like depressions where the DNA samples are positioned.
To prepare for the addition of DNA samples, the gel needs to be inserted into a gel box. The gel box consists of two ends, one connected to a positive electrode and the other to a negative electrode. The central region of the box, where the gel is placed, is filled with a buffer solution containing salts that facilitate the conduction of electrical current. Although not visible in the provided image, the buffer solution fills the gel box to a level where it only covers the gel slightly. The end of the gel that contains the wells is oriented towards the negative electrode, while the opposite end, where the DNA fragments will migrate, is positioned towards the positive electrode.
Gel electrophoresis utilizes a gel, which can be described as a solid, gelatinous material resembling Jello. In the context of DNA separation, agarose, a polysaccharide, is commonly used to create the gel. Agarose is initially in the form of dry, powdered flakes and is then mixed with a buffer solution (water with salts) and heated. As it cools, the agarose solidifies into a slightly soft gel. On a molecular level, the gel consists of a network of agarose molecules interconnected by hydrogen bonds, forming small pores. The gel features wells, which are pocket-like depressions located at one end, and these wells are where the DNA samples are placed for analysis.
Firstly, the gel needs to be prepared for DNA sample application by being inserted into a gel box. The gel box consists of two ends, with one end being connected to a positive electrode and the other end connected to a negative electrode. Within the main body of the box, a conductive buffer solution containing salts is poured, which allows for the flow of electric current. Although it may not be clearly depicted in the image (due to my extraordinary artistic abilities), the buffer solution is carefully added to the gel box until it reaches a level that just covers the gel. It's important to note that the end of the gel containing the wells is oriented towards the negative electrode, while the opposite end, where the DNA fragments will migrate, is positioned towards the positive electrode.
After placing the gel inside the gel box, we carefully introduce each of the DNA samples we wish to analyze (such as PCR reactions or restriction-digested plasmids) into individual wells. Additionally, one well is specifically designated for a DNA ladder, which serves as a reference containing DNA fragments of known lengths. It is advisable to select a commercial DNA ladder that covers the expected size range of our fragments adequately.
Subsequently, the power supply to the gel box is activated, initiating the flow of electric current through the gel. Due to the presence of negatively charged phosphate groups in the sugar-phosphate backbone of DNA molecules, they commence their migration through the gel matrix towards the positive electrode. This movement occurs when the power is switched on, leading to the gel being referred to as "running" as current passes through it.
During the gel running process, DNA fragments of shorter lengths exhibit a higher velocity as they traverse through the pores within the gel matrix compared to longer fragments. As time progresses and the gel continues to run, the shortest DNA fragments will approach the positive end of the gel, while the longest fragments will remain closer to the wells. It is worth noting that extremely short DNA fragments may have migrated beyond the gel if the running process was extended for an excessive duration.
To visualize the separated DNA fragments, we can analyze the gel and observe the bands of various sizes present on it. By treating the gel with a DNA-binding dye and exposing it to ultraviolet (UV) light, the DNA fragments become fluorescent, enabling us to visually identify the DNA's presence at different positions along the gel's length.
A distinct segment of DNA that appears as a clearly defined mark on the gel is referred to as a band. Within each band, numerous DNA fragments of identical size have collectively migrated to the same location. It is important to note that a solitary DNA fragment or a small cluster of fragments would not be individually visible on the gel.
By conducting a comparison between the bands observed in a sample and the DNA ladder, we can estimate their approximate sizes. For instance, the prominently visible band depicted in the above gel is approximately X base pairs (bp) in length.
Horizontal gel electrophoresis is a technique that employs the fundamental principles of separating DNA, RNA, or protein molecules based on their molecular size and charge. In this method, the gel is positioned horizontally and submerged in a continuous buffer. An agarose gel is utilized to divide the gel box into two compartments. One end of the gel box contains an anode, while the other end contains a cathode. When an electric current is applied, the buffer enables the creation of a charge gradient. However, the application of a load causes the gel to heat up.
The buffer also serves as a coolant, maintaining the temperature at optimal levels. By recirculating the running buffer, the formation of a pH gradient is prevented. Horizontal gel electrophoresis cannot employ a discontinuous buffer system since the two compartments of the gel system are connected to the running buffer.
In horizontal gel electrophoresis, acrylamide cannot be used because the gel box is exposed to oxygen. The presence of oxygen inhibits the polymerization of acrylamide, which hampers gel formation. Horizontal gel electrophoresis is a straightforward technique commonly employed for separating DNA and RNA.
Vertical gel electrophoresis operates based on the fundamental principles of gel electrophoresis, but it is considered more intricate compared to the horizontal gel electrophoresis method. This technique utilizes a non-continuous buffer. The upper chamber contains a cathode, while the lower chamber contains an anode. The electrodes in each compartment generate the necessary electric field. A thin layer of gel is poured between two glass plates, with the upper part of the gel immersed in the upper chamber and the lower part immersed in the lower chamber. When an electric current is applied, a small portion of the buffer moves from the upper chamber to the lower chamber through the gel.
In vertical gel electrophoresis, the buffer solely flows through the gel, enabling precise control of the voltage gradient during the separation process. Acrylamide gel can be used in this technique as the compartments are not exposed to atmospheric oxygen. The smaller pore size of the acrylamide gel allows for precise separation with higher resolution.